Malic Acid in Sugar Confectionery
Jump to…
Malic acid was first isolated from apples in 1758. It has been used as a food acidulant for about 40 years and has the E-number E296 in Europe. Although it occurs naturally in many fruits, malic acid is usually manufactured by chemical synthesis on a commercial scale as this is the most economical method available.
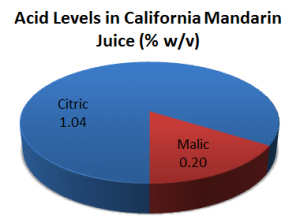
Because fruits contain a mixture of acids rather than any one single acid, the taste profile of many fruit-flavoured confections can be hugely improved by copying nature and using a blend of acidulants. For example, the primary acid in mandarin juice is citric acid and is present at a level of 1.04% (w/v). Malic acid is the secondary acid and makes up 0.2% (w/v). There are traces of other acids too.
Malic acid provides a clean and mellow flavour without astringency. It can enhance fruit and mint flavours especially. Malic acid’s slightly hydrophilic nature (attracted to water) means that it will associate with non-polar components of the product or saliva. Association delays flavour impact and gives an overall blended and therefore more natural flavour profile.
In a taste test of an artificial-orange flavoured beverage, it was found that a mixture of citric acid and malic acid in a ratio of 50:50 was preferred over 100% citric acid. Malic acid balances the sweetness and persistence of aspartame and gives a “zesty”, more natural flavour.
Acidulants can react with the sugar in hard candies, converting it from hard sucrose into gooey glucose and fructose. This process is known as inversion. The rate of inversion depends very much on the number of hydrogen ions present from dissociated acid. Usually the moisture level in hard candy is between 1% and 3% so the amount of acid able to dissociate is small and the process happens slowly. Nevertheless, steps can be taken to prevent this from happening.
Sucrose inversion is accelerated at high temperatures. Adding the acid last minimizes the exposure to high temperatures. Adding the acid in granular form reduces the moisture level in the final product. Malic acid especially has a lower melting point than other granular acids and so mixes easily with the molten hard candy. Using malic acid and/or a buffer salt results in a less acidic final product which slows inversion. Finally, it is possible to sand the candy with malic acid coated in unreactive vegetable fats to prevent contact with the sugar instead of adding the acid to the candy directly.
Gummy sweets made with gelatin have a very pleasing mouthfeel. The gelling power (or bloom) becomes stronger as acidity decreases; however adding an acidulant to raise the level of acidity is favourable for improving the microbiological stability of the product without the need for preservatives.
As with hard candies, the malic acid is added last to prevent the gum being hydrolysed during the process. These softer sweets often include fat or starch which can either mask or delay the impact of many acidulants. Malic acid’s persistence avoids this.
Malic acid is used in confectionery products because it is a multi-functional and versatile ingredient. From creating sourness, to improving and blending fruit flavours, to altering the physical properties of the candy, malic acid can even save on the cost of other ingredients.
Acidulants for Confectionery Products, presentation by Dan Sortwell, June 1998
Malic Acid in Citrus Fruits, Technical Bulletin, Bartek Ingredients Inc.